So, we could not finish the covalent solids in the last lecture.
So, we will do the remaining part here, and then we will continue with the ionic solids, the introduction to ionic solids. So, we were talking about the formation of covalent solids, and we talked about why hybridization is important because the orbital overlap is not sufficient to explain why certain kind of bonding occurs? Why covalent bonding occurs with four neighbors in solids like methane and silicon and carbon, and that is where the concept of hybridization came.
So, we are looking at the structure of graphite, which is the SP2 bonded material, in which carbon atoms are SP2 hybridized and bonded in the plane. So, it makes a hexagonal sheet, but the sheets are themselves bonded to each other with Vander Waals.
or secondary bonding, and that is why the interaction between the sheets is weaker, and so when you apply force to graphite the sheets get sheared very easily. As a result, graphite is used as a lubricant. Second form of carbon that we going to talk about is diamond, which is again a form of carbon.
So, for example in the case of diamond what happens is that if you make a unit cell of diamond, the carbon atoms are arranged on the face centers of a cube and then carbon atoms are arranged in such a fashion, so they are also present at the tetrahedral sites, but not all tetrahedral sites are present otherwise you will violate the co covalent bonding.
So, carbon atoms are present in such a fashion you have a carbon atom here which is covalently bonded with respect to this and the one here and the one here, so they will make a tetrahedral. So, it will make a bond here, again it will make four bonds. So, this is first neighbor, this is second neighbor, this is third neighbor, and this is fourth neighbor. Similarly, you will have one atom here just across the phase diagonal, other two carbon atoms do not go in the bottom side, they go here. So, one carbon atom goes here, and one carbon atom goes here. If you put carbon atom on all the tetrahedral sides, then some carbon atoms will end up having eight neighbors, and that is not permitted by SP3 hybridization and bonding. So, as a result, you have to arrange the carbon atoms in such a fashion so that you have a tetrahedral coordinated structure. So, let me show you one viewgraph of this structure how it looks like.
And same is true about the carbon atom sitting at the corners and face centers they are also tetrahedrally coordinated because if you if you keep the unit cell corner from dark gray to light gray, then what you will see is that the light gray will make an FCC unit cell, and that dark gray will occupy the tetrahedral voids. So, the diamond cubic structure is two interpenetrating FCC lattices shifted to each other with respect to a vector ¼ ¼ ¼.
So, this is the structure you can see this is the top view, which makes the original lattice FCC lattice, and these are the tetrahedral ones ¼ ¾ ¼. Now you can make the unit cell by placing the by shifting the unit cell a little bit, I can make the unit cell here. So, you will make FCC lattice, and these atoms will become this will become a tetrahedral atom, this will become a tetrahedral atom, this will become a tetrahedral atom, and this will become a tetrahedral atom. So, basically these are two interpenetrating FCC lattices into each other shifted by a vector ¼ ¼ ¼, and this lattice is called as diamond cubic lattice, and this is followed by diamond, it is followed by silicon, but it is also followed by silicon carbide, zinc sulfide.
So, what happens in silicon carbide is that in silicon carbide, this atom will be silicon, and this atom will be carbon. Similarly in case of zinc sulfide this would be zinc, this would be sulfur; you can make this as zinc, and this sulfur both are possible representations, but zinc sulfide a slightly ionic character. So, that is why we will discuss the structure of zinc sulfide in case of ionic solids, but diamond silicon carbide or even silicon has this kind of structure, which is called as diamond cubic lattice.
But diamond cubic lattice is not a Bravais lattice, it is still a face-centered cubic lattice, but the motif is different motif is face-centered cubic lattice, 0 0 0 and ¼ ¼ ¼ will be the motif because you have two atoms. So, it is a lattice which contains eight atoms you can calculate the packing fraction, and you can see that the diamond atom is sitting carbon atom is sitting as a tetrahedral site is 0.225r, and you have the same sized atom sitting there which means the lattice has to dilate.
So, lattice of silicon or carbon is bigger than what it would have been, if there was no tetrahedral atom, of course, that is not possible bonding wise, but imagine if carbon only made FCC lattice then the packing fraction would be same as 0.74, but in this case packing fraction would be lower because you have to dilate the lattice. So, you can calculate the packing fraction by yourself.
This is the structure of diamond, this is the structure of another covalent material called C60, which is the fullerene buck ball kind of structure. So, it has a football-shaped structure where carbon atoms are arranged in such a fashion. So, that you see these truncated, you see these hexagons and pentagons arranged. So, you can see that if you only had pentagons it will not make a structure, but if you put pentagons separated by hexagons then it can make a ball kind of structure. So, this is a C60 molecule, which is called as fullerene, and the structure of this is again a covalently bonded materials you can see that each atom having three neighbors here SP2 hybridized structure.
And then there are some other covalent solids, these are the ones which I discussed in the beginning.
So, for example, iodine makes a structure like this, so iodine is an orthorhombic structured material has linear 1-fold coordination. So, these are iodine atoms, this is one I, this is second I, so it has only one neighbor. So, dumbbell-shaped molecules arranged in an orthorhombic lattice. This is structure of Tellurium, which is a spiral structure, it is a hexagonal it again has 2-fold coordination in a spiral network.
So, these are the covalently bonded structured materials that we wanted to discuss in this lecture. So we have other examples are silicon, silicon carbide, zinc sulfide similar structures. So, you can say structure is diamond cubic lattice, not a Bravis lattice, very important to remember.
So, Bravis lattice is FCC with a motif at 0 0 0 and ¼ ¼ ¼. So, it is a mixture of two interpenetrating FCC lattices, in case of silicon carbide, zinc sulfide there will be 2 FCC lattices, one of silicon and one of carbon, similarly, in zinc oxide, it will be one of zinc and one of sulfur.
So, now we will start the discussion on ionic solids and this discussion is important because, many compounds that we see around are all ionically bonded materials and for example, if you see some bonds like sodium chloride, potassium chloride, several oxides nickel oxide, copper oxide, iron oxide compounds such as barium titanate and alumina, even zinc oxide they are all ionically bonded materials, but they may not be 100% ionic because ionic bond forms when there is a large difference between the electronegativity of the two atoms.For example, in the case of sodium, you have lithium fluoride. When the elements have large difference in the electronegativity, the bond is predominantly ionic. In some cases, when that the difference is not large, the bond may be partly covalent, in most cases, it is partly covalent and partly ionic, but they fall in the category of predominantly with ionic character.
So, bonding, in this case, is bonding is predominantly ionic exceptions that may have covalent character. So, for example, zinc oxide, zinc sulfide, silicon carbide and all that. So, percentage iconicity or ionic bond is proportional to difference in the electronegativity of let us say A and X, where A is cation X is anion.The periodic table, on the left, we have let us say 1A then we have 2A, and in 1A, we start with hydrogen, lithium, and we have sodium then we have potassium and so on. Lithium has an electronegativity of 1.0, and sodium has a potassium electronegative 0.9, potassium has electronegativity of 0.8. In the second one, we start with beryllium, magnesium, calcium. Magnesium has electronegativity of 1.2, calcium has a negative electronegativity of nearly 1 and then, of course, you go to Group-IV, we have atoms like carbon, silicon, and bismuth, and all that to carbon has an electronegativity of 2.5, silicon has electronegativity of 1.8, and then an important one is again group-VI, where you have oxygen, which has an electronegativity of 3.5, and then you have sulfur which has an electronegativity of 2.5, and then we have fluorine which has an electronegativity of 4, chlorine has an electronegativity of 3 and so on.
But when you mix these compounds, for example, lithium and fluorine, it is a large difference in the electronegativity, if you mix chlorine and sodium, it is a large difference between the electronegativity. If you mix, for example, potassium with chlorine, you can have a large difference in the electronegativity, you can mix magnesium with oxygen, calcium with oxygen these all have very large differences in the electronegativity, and these all give you large ionicity in the bonding.
So, for example, like calcium fluorite, lithium fluoride will give you a largely ionic bond, but on the other hand, if you silicon carbide, silicon, carbon, the difference is hardly any difference 1.8, 2.5. So this is predominantly covalent. Similarly, if you zinc oxide, zinc sulfide, zinc is is just before column three. So, this is zinc, zinc has an electronegativity of 1.8, zinc oxide is more ionic, but zinc sulfide is less ionic, and zinc sulfide is more covalent in nature.
So, this is the the the the reason why you have this variety of compounds showing predominantly ionic behavior compound showing predominantly covalent behavior and compound showing mixed behavior, but all of them they have certain ionic character that is why they fall in the category of ionic solids. So, first before we go into how ionic solids form let us first look into what makes ionic structures, what makes them stable.
So, when you put several anions and cations together, they form these structures which are periodic in nature, they have certain crystal structures the regular periodicity rather than making isolated molecules.
Why cannot you have one silicon carbide or maybe one zinc oxide molecule, other zinc oxide molecule, then why do they have to arrange themselves in a periodic fashion, why cannot just you have zinc oxide molecule that is it. So, you can have several zinc oxide molecules that are randomly located in their space now, this is to do with the energetic.
So, here if you a the cations are defined by the energy that we consider, in case of cations is ionization energy right, energy required to ionize them. In case of anions what we consider is electron affinity because they are ready to accept the electron that is why we talk about electron affinity. The energy of an ionic bond is determined by when you put these atoms together you have the columbic attraction, and you have repulsion, repulsion is often called as poly repulsion.
So, when you put A and X, this is A+ X-, there is a tendency to attract, but suppose you have AX molecule, you have another AX molecule in the vicinity. Suppose if it was in this position it would tend to attract, suppose you have AX, and the neighboring molecules are oriented in such a fashion so that XA is coming closer, this will tend to repel. So, the balance of these attractive and repulsive forces eventually leads to a structure that is periodic in nature, that is why the molecules are not randomly just arranged because if molecules are randomly arranged you will have A close to each other in some cases you will have AX closing. So, you have to have balance the forces. So, basically the energy expression of this structure is,
Where, B is a constant, and n takes a value of about 10. So, for every Jth neighbor, you got to sum this energy and multiply by n, which is a number of molecules in the system. So, this will eventually give you a energy landscape. So, basically you have two terms here first term goes as minus of 1 over r and the second term goes as r to the power n. So, this is this is the force which is attractive in nature, and this is a force which is repulsive in nature, it is the balance of these two forces which will determine whether you will have a minima in energy for a structure when you arrange the atoms in a periodic fashion. So, there are multiple possibilities for which the energy is minimum.So, for example, sodium and chlorine together, let us say you have Na+ and Cl- when you bring them together you form NaCl bond. So, this is basically when you bring them together you spend energy. So, you spend energy to bring them together and what is that energy? which is ionization energy, Ei and electron affinity, χ. So, it is the difference between Ei and χa, this is the amount of energy I need to spend in creating a bond. The second is the energy gained is called cohesive energy, which is achieved by bringing the atoms together. For example, this is a sodium atom, this is chlorine and so on and so far you will have this n symbol of ions in this fashion.So, basically you arrange them in a periodic fashion it is not an exact representation, but I understand I hope you understand what I mean. So, this is not a right way to do it let me just show you. So, the bottom one plane would be something like that, and this is chlorine atom; these will be sodium atoms, these are all sodium these are all. So, you put them together when you bring them together you gain energy in the form of cohesive energy, and you need to worry about whether there is a net attraction or not. If there is a net attraction then the structure is stable if there is no attraction it says more repulsion structure is not stable.
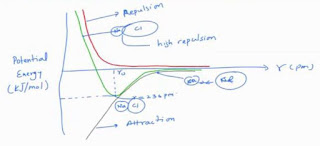
So, the overall curve of the energy landscape would look like that. So, if you draw, this is potential energy and typically in kJ/mol, and this is the distance r. So, your repulsion term goes rather rapidly, something like that very rapidly. This is the repulsion, and then you have that total energy, and this would be the term which is this is attraction, and this is what is keep going to give you the minima. So, this is the potential energy minima. This is the equilibrium separation, which is nearly 236pm approximately. You can see that higher distances give you higher energy. So, at this distance you have lower energy, so this is in r in picometer, and at very low values you have very high repulsion.
So, if you go lower than 236, your deposition increases, and if you go higher 236, then again it although it is attractive, the energy is not minimum, the energy tends to increase. So, this is the point at which you hang around to it, and so if you talk about very large small distances it is like this silicon and chlorine sodium, and chlorine is squashed together really very close, this will mean high repulsion, and if you come somewhere here then it would mean situation like NaCl. So, they will tend to attract, but then they are too far, so they become like isolated atoms.
So, this is the energetics of ionic solids, and we will discuss the details of this energetics a little bit more in the next class. And also we will discuss Madeleine constant, which is a useful term to know in the context of ionic solids and then we will see there are certain rules which one needs to know to form the ionic structures which are called as Pauling rules and we discuss the structure of some of the common ionic solids.
For the Previous Lecture Click below
Thank you very much
Click Here : Please Subscribe, Like and Share my channel











Comments
Post a Comment